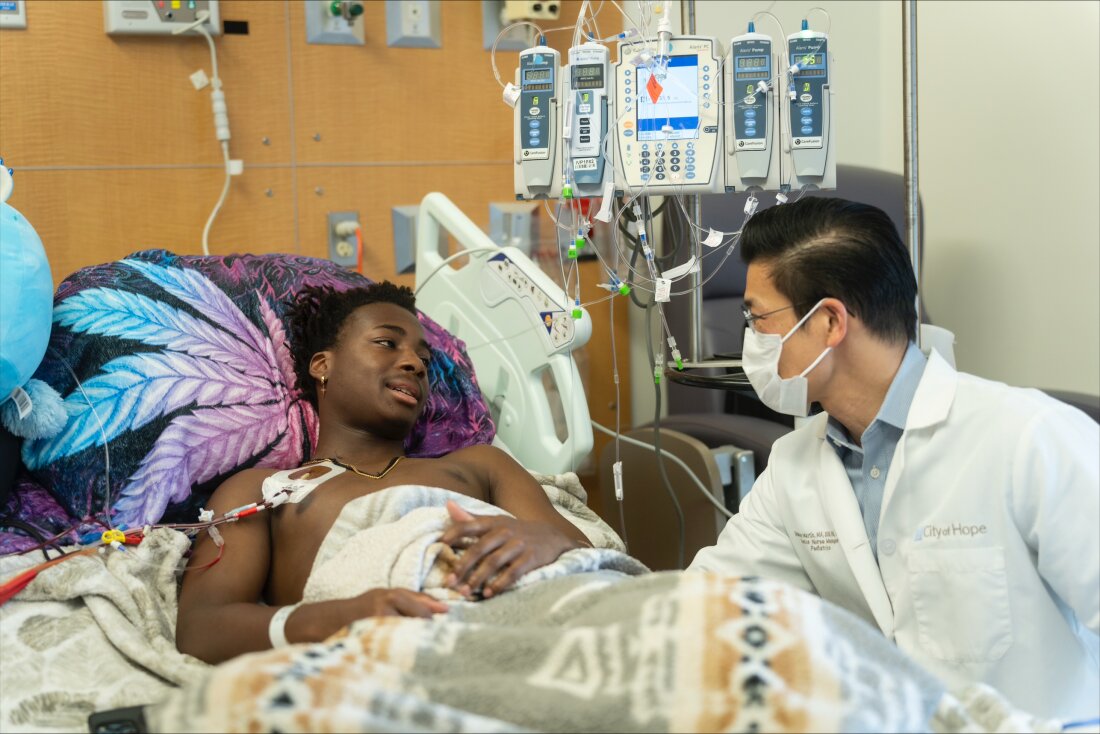
As his stem cells are collected, DeShawn “DJ” Chow discusses his upcoming sickle cell gene therapy with pediatric hematologist Leo Wang at City of Hope Children’s Cancer Center on 22 May.
City of Hope
hide description
toggle caption
City of Hope
Olaide Adekanbi has been battling sickle cell disease all her life.
Adekanbi, 29, who lives in Boston, says: “It’s like I’m fighting. “It’s like darkness, I don’t know if you can call it evil inside, [but] sometimes it feels that way [it].”

This rare genetic blood disorder is caused by a genetic mutation that causes the red blood cells to become deformed, sickle-shaped. These malformed cells clog blood vessels, damage vital organs and cause unexpected, debilitating pain attacks.
He says: “Sometimes you get to the point where you feel like, ‘I can’t go on living like this.’ “You feel like you’re losing your mind. Because sometimes I can’t move. I just lie in one place and try to ignore the pain I feel.”
So Adekanbi was delighted when, in late 2023, the Food and Drug Administration approved the first gene therapy for sickle cell, a disease that disproportionately affects Black people like him and has been largely ignored by medical science.
He says: “I am very happy with the opportunities. This is probably the best time in history right now for sickle cell patients.
Risks and unknowns complicate the decision
But Adekanbi isn’t sure if he wants to go ahead with one of the two approved gene therapies.
The biggest setback is because of the chemotherapy needed to replace the genetically modified cells in his brain. Those cells have been modified to alleviate the symptoms of the disease. But chemotherapy would jeopardize her chances of having children.
“I know I would like to have children in the future,” she says. “So I’m really worried about the process that your body goes through in order to be able to go through the gene therapy process – how that will affect fertility.”
Ms. Adekanbi is not alone in wondering what to do. Although there is great excitement about treatments for sickle cell patients and those with a related disease known as beta thalassemia, only about 60 of the thousands of patients eligible for treatment have started the process. this one.
Adekanbi says she will try to freeze some of her eggs if she decides to go ahead. But she and other potential patients are concerned about going beyond their conception. Treatments are also difficult and complicated in some ways.
Melissa Creary, who studies sickle cell at the University of Michigan School of Public Health, says: “You can be in the hospital for months. “Even if you are not in a hospital, you will have to be close to a hospital, which would be in the situation where you live or it would not be there. Then once the treatment is over, there is a complex follow-up process. – for many, many months, and you’re in a state where you don’t live.”

While Olaide Adekanbi is undecided about pursuing sickle cell gene therapy, she is excited about the options. “This is probably the best time in history right now for sickle cell patients,” he says.
Olaide Adekanbi
hide description
toggle caption
Olaide Adekanbi
The treatments are very expensive, costing between $2.2 million and $3.1 million per patient.
“Cost continues to be a major barrier … in terms of getting it to the people who need it most,” Creary says.
And some patients worry about potential long-term risks, according to Dr. Lewis Hsu, chief medical officer of the Sickle Cell Disease Association of America.
“What will happen to me in 10 to 20 years? Will I fail using genetics? They will not persist in my system for that long? Or will there be a second leukemia? Hsu said. “We don’t have good information yet because nobody has been out that long.”
For their part, Vertex Pharmaceuticals of Boston and Bluebird Bio of Somerville, Mass., which makes the treatments, say both treatments appear to be safe so far.
And while it’s not surprising that it takes time to find treatments that are widely accepted, given how complex and expensive they are, both companies say profits are increasing quickly.
More hospitals have signed on to offer the treatment, and more are coming online every day, the companies say.
In addition, companies are working to help patients pay for treatments and related care, and many public and private insurances cover it.
“We’re seeing a lot of activity based on what we thought would be the level of interest. So we’re very encouraged by what we’re seeing,” says Andrew Obenshain, chief executive officer of Bluebird Bio. “Hospitals have been built and are ready for treatment. Those who pay pay for it. And patients are interested.”
Bluebird’s opponent agrees.
“It is really a very important decision for the patient to go on this journey. But I’d say it’s going well,” says Stuart Arbuckle, vice president and chief operating officer at Vertex. “The response we’ve had from payers, physicians and patients has been very gratifying. ”
Economic inequality can limit access
But finding all the costs can be difficult. And it remains unclear how many patients with the genetic disease will find them, as they live in economically poor countries in places like Africa and Asia where new treatments remain elusive.
“We’ve done something that’s almost useless to people,” says Rimas Orentas, chief science officer at Caring Cross, a nonprofit group that advocates for greater access to new medical technology. many suffer from sickle cell disease.”
That worries Victoria Gray of Forest, Miss., the woman who was the first person with sickle cell to receive gene therapy. NPR broke the news when Gray was treated in 2019.
“A lot of people are suffering — not just suffering, but dying — every day,” says Gray, who is 39 and works full-time at Walmart. “And we have something now to stop it. I want people to be free from this kind of fear, anxiety and a state of unspeakable pain.”
One of the patients on that road is DeShawn Chow, 19, of Irvine, Calif. He began treatment at City of Hope Children’s Cancer Center in Los Angeles earlier this year. Her insurance covers the treatment, and she is not concerned about the effect it may have on her ability to have children.
“The first time I heard about it I wasn’t sure about it,” Chow says. “But I hope it will change my life.”
#Gene #therapy #sickle #cell #disease #starts #slowly